Carbon-14
For some reason which I
Carbon-14
 For some reason, which I have not yet figured out, at least one person per week has been asking me about the Carbon14 dating technique. They want to know if it is accurate or if it does work. Worse still, they want to know how evolutionists use Carbon14 to date dinosaur fossils!
For some reason, which I have not yet figured out, at least one person per week has been asking me about the Carbon14 dating technique. They want to know if it is accurate or if it does work. Worse still, they want to know how evolutionists use Carbon14 to date dinosaur fossils!
The Carbon14 "dating" method was introduced by Dr. W. F. Libby at the University of Chicago in 1948. He claimed that it was capable of dating animal, vegetable and human remains of fairly "recent' origin. Recent, that is, for an evolutionist. Carbon14 is a long-lived radioactive isotope of Carbon. Carbon12 is the normal stable isotope of Carbon, which is the basic building block of organic life forms. As they say on Star Trek, we are all carbon based units.
Laboratory research has shown that the radioactive decay of Carbon14 occurs in a half-life of 5,730 years. That means that starting with one pound of 100% Carbon14, half of it would decay in 5,730 years, leaving 50%, or half a pound. Then, in another 5,730 years, a second decay period would occur, leaving one quarter of a pound. The process would continue, halving the amount left every 5,730 years until, theoretically, nothing remained of the original pound.
Carbon14 is produced in the upper atmosphere through the bombardment of Nitrogen (approximately 80% of atmospheric gases) by neutrons which come from the powerful cosmic radiation, primarily generated by the sun. This bombardment causes a nuclear reaction to take place. The Carbon14 produced by this process is then converted into Carbon Dioxide, just as normal Carbon12 is becomes Carbon Dioxide. The Carbon(14)Dioxide is then utilized by plants during their normal metabolism. Animals and humans who eat these plants take the Carbon14 into their systems just as they would Carbon(12)Dioxide.
There is then a ratio of Carbon14 to Carbon12 in the bodies of plants, animals and humans which could be considered as "fixed" at the time of death. After death, the Carbon14 would decay and the ratio of the two isotopes would change. Evolutionists then claim to determine the amount of time since the death of the organism by measuring this ratio. The lower the amount of Carbon14, the longer it has been since death occurred.
As you can see, the theoretical limit of the usefulness of Carbon14 would only be about 50,000 years. This would be the amount of time it would take for nine half-lives, and after that there would not be enough left to measure accurately. There are, however, many assumptions which have led to serious flaws in this supposedly useful method.
First, one must assume that the decay rate of Carbon14 has remained constant and not varied over the years. This is an unwarranted assumption. There is evidence to indicate that quite the opposite is true. Experiments done with the radioactive isotopes of Uranium238 and Iron57 have shown that rates not only do vary, but can, in fact, be altered by changing the environment surrounding the samples.
Second, there is the assumption that the formation of Carbon14 has been constant throughout the years. This, too, is a totally unwarranted view for two reasons. The Industrial Revolution caused a significant increase in the amount of Carbon12 in the atmosphere through the burning of coal. In addition, the initiation of atomic bomb testing on July 16, 1945, and the subsequent above ground testing that followed, caused a rise in neutrons which in turn increased Carbon14 concentrations around the world.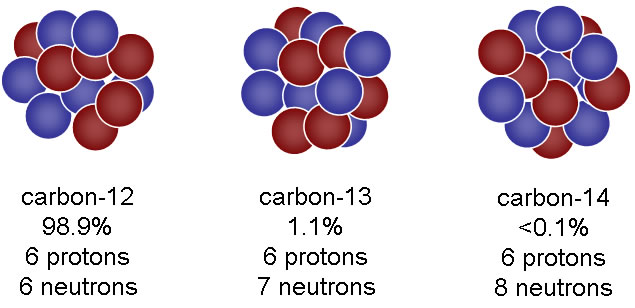
Third, the assumption is made that the concentrations of Carbon14 and Carbon12 have remained constant in the atmosphere. Besides the aforementioned items, the amount of cosmic radiation in the past, and in particular the amount reaching the atmosphere, may have been dramatically different. If one were to believe the Bible, the earth was surrounded by a layer of water vapor between Creation and the Flood. If this water vapor did exist in the past, then it would have effectively shielded the atmosphere from cosmic radiation. This shielding would have drastically reduced the amount of Carbon14 produced.
Fourth, Dr. Libby assumed that the amount of Carbon14 being produced in the present had reached equilibrium with the amount of Carbon12; he assumed that the two were in balance with each other. Since Carbon14 is a radioactive element, it starts to decay immediately upon formation. If you start with zero Carbon14 in the atmosphere, it would take 50,000+ years for the amount being produced to equal the amount decaying. One of the reasons that we know the earth is younger than 50,000 years old is that the amount of Carbon14 in the environment is only 78% of the amount that it should be, if the earth were old.
Fifth, evolutionists have assumed that all plants and animals take in and utilize Carbon14 equally in their systems as they do Carbon12. Quite the contrary is true! Mollusks found alive off the coast of Hawaii have had their shells dated with the Carbon14 method. The shells were calculated to have died 2,000 years ago, yet the animals were still alive. Natural crude oil has been dated using the Carbon14 technique; yet evolutionists would teach that oil is too old to be dated using Carbon14. The grass used as part of the mortar in an old English castle, known to have been built only 800 years ago, was dated by Carbon14 to be thousands of years old. The list of non-compliant dates derived by Carbon14 is literally endless.
All of these assumptions have caused there to be considerable overestimates using the Carbon14 technique. Today, most evolutionists, including Dr. Libby, would agree that the method is at best usable for only a few thousand years.
In 1970, Dr. R. L. Whitlaw, collected a database of 15,000 Carbon14 dates attributed to various plant, animal and human remains. He grouped these three categories and then studied the patterns they formed. He found a number of significant points.
Whitlaw noticed that the number of human and animal remains increased from 7,000 years ago to 5,000 years ago. At that point there was an abrupt decrease in the populations to one-tenth the previous amount, followed by a slow and gradual increase to the present. He also noticed that the populations of creatures in North and South America lagged behind the population numbers in Africa and Europe.
While I would disagree somewhat with the exact dates he attributes to the samples, both of his observations would be consistent with the biblical record. The Bible paints a picture of life initially originating in the Middle East and migrating across the world. This migration and growth in population would then be punctuated by the death of most air-breathing land-dwelling creatures in the Flood of Noah. After the Flood we would expect to see the same migration and population growth patterns develop for a second time.
Perhaps the best description of the problem in attempting to use the Carbon14 dating method is to be found in the words of Dr. Robert Lee. In 1981, he wrote an article for the Anthropological Journal of Canada, in which stated: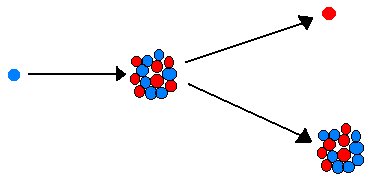
"The troubles of the radiocarbon dating method are undeniably deep and serious. Despite 35 years of technological refinement and better understanding, the underlying assumptions have been strongly challenged, and warnings are out that radiocarbon may soon find itself in a crisis situation. Continuing use of the method depends on a fix-it-as-we-go approach, allowing for contamination here, fractionation there, and calibration whenever possible. It should be no surprise, then, that fully half of the dates are rejected. The wonder is, surely, that the remaining half come to be accepted.
No matter how useful it is, though, the radiocarbon method is still not capable of yielding accurate and reliable results. There are gross discrepancies, the chronology is uneven and relative, and the accepted dates are actually the selected dates."
There you have it! The Carbon14 'dating" method is as invalid as the evolutionary philosophy for which it was introduced and supposed to support!
Last updated: 19th November 2012 by http://www.creationworldview.org/articles_view.asp?id=6

